Cell Metab, May 2022
Garreta Elena, Prado Patricia, Stanifer Megan L, Monteil Vanessa, Marco Andrés, Ullate-Agote Asier, Moya-Rull Daniel, Vilas-Zornoza Amaia, Tarantino Carolina, Romero Juan Pablo, Jonsson Gustav, Oria Roger, Leopoldi Alexandra, Hagelkruys Astrid, Gallo Maria, González Federico, Domingo-Pedrol Pere, Gavaldà Aleix, Del Pozo Carmen Hurtado, Hasan Ali Omar, Ventura-Aguiar Pedro, Campistol Josep María, Prosper Felipe, Mirazimi Ali, Boulant Steeve, Penninger Josef M, Montserrat Nuria,
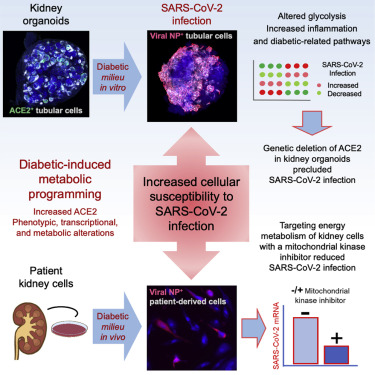
A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells.
It is not well understood why diabetic individuals are more prone to develop severe COVID-19. To this, we here established a human kidney organoid model promoting early hallmarks of diabetic kidney disease development. Upon SARS-CoV-2 infection, diabetic-like kidney organoids exhibited higher viral loads compared with their control counterparts. Genetic deletion of the angiotensin-converting enzyme 2 (ACE2) in kidney organoids under control or diabetic-like conditions prevented viral detection. Moreover, cells isolated from kidney biopsies from diabetic patients exhibited altered mitochondrial respiration and enhanced glycolysis, resulting in higher SARS-CoV-2 infections compared with non-diabetic cells. Conversely, the exposure of patient cells to dichloroacetate (DCA), an inhibitor of aerobic glycolysis, resulted in reduced SARS-CoV-2 infections. Our results provide insights into the identification of diabetic-induced metabolic programming in the kidney as a critical event increasing SARS-CoV-2 infection susceptibility, opening the door to the identification of new interventions in COVID-19 pathogenesis targeting energy metabolism.
Visit Journal