Communication Biology, Aug 2022
Pasquale Laise , Megan L Stanifer , Gideon Bosker , Xiaoyun Sun , Sergio Triana , Patricio Doldan, Federico La Manna , Marta De Menna , Ronald B Realubit , Sergey Pampou , Charles Karan, Theodore Alexandrov , Marianna Kruithof-de Julio , Andrea Califano , Steeve Boulant , Mariano J Alvarez
A model for network-based identification and pharmacological targeting of aberrant, replication-permissive transcriptional programs induced by viral infection
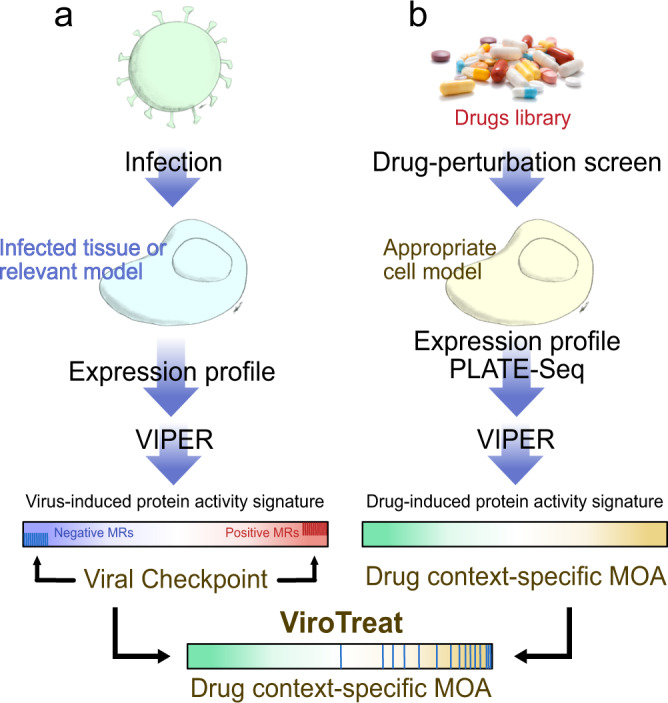
SARS-CoV-2 hijacks the host cell transcriptional machinery to induce a phenotypic state amenable to its replication. Here we show that analysis of Master Regulator proteins representing mechanistic determinants of the gene expression signature induced by SARS-CoV-2 in infected cells revealed coordinated inactivation of Master Regulators enriched in physical interactions with SARS-CoV-2 proteins, suggesting their mechanistic role in maintaining a host cell state refractory to virus replication. To test their functional relevance, we measured SARS-CoV-2 replication in epithelial cells treated with drugs predicted to activate the entire repertoire of repressed Master Regulators, based on their experimentally elucidated, context-specific mechanism of action. Overall, 15 of the 18 drugs predicted to be effective by this methodology induced significant reduction of SARS-CoV-2 replication, without affecting cell viability. This model for host-directed pharmacological therapy is fully generalizable and can be deployed to identify drugs targeting host cell-based Master Regulator signatures induced by virtually any pathogen.
Visit Journal